Posts Tagged hdls
SLAM and Drones and HDLS….oh my
Posted by Jim Foster in Uncategorized on October 5, 2018
SLAM and Drones and HDLS….oh my.
There seems to be new tech, and “reality capture” methods popping up on the daily. The lens that I look at it through though is how can any of the technologies and methods get us to a point quicker and more accurately. No, we’re not in Kansas anymore.
SLAM (simultaneous localization and mapping ) , these devices and specifically the ones developed by GeoSlam, was the first time I bought a demo and did not want the rep to leave my office with what he brought. The conversation went something like this “I’ll take it”. “Okay we can write you up and deliver….” “No I’ll take what you have in your hands right now, how much” and subsequently we bought another. As far as the combination of speed, cost and quality (granularity of data) I have found it pretty remarkable in what it does. It has its limitations though and why we augment SLAM data with other collection methods to get us to a better whole, and again; this depends on the scope of the project.
Many of you, certainly if you are reading this, are familiar with HDLS (High Definition Laser Scanning) which most people just refer to as LIDAR . Why make the distinction? Because LIDAR really is the big tent, laser scanning as a whole, and all the machines, processes are the acts. HDLS typically requires individual set ups, the machines stay stationary and the process while slower and more cumbersome still produces the highest density of data. To help explain this I’ve come up with possibly a slightly less confusing metaphor or perhaps no help at all. Steam, water and ice. With steam water in the gas phase and it’s molecules are far part, relatively speaking, water, liquid phase are tight, and ice, water in solid form the molecules are tighter yet. With scanning the density of the points, like water molecules are tighter or farther apart based on the technology you are using and why we need to use different ones based on the scope of the project.
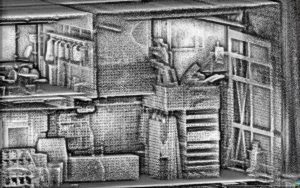
 In the images I have included, the first one is created through HDLS using tripod mounted stationary FARO Focus. If you zoom in, you can see the handles on the cabinets, the images in the painting, etc, this is because the machine takes photos as part of the process, at least in this case) and maps them to the collected points, giving us a true 3D representation of the space, which is the back of a theater by the way.
In the images I have included, the first one is created through HDLS using tripod mounted stationary FARO Focus. If you zoom in, you can see the handles on the cabinets, the images in the painting, etc, this is because the machine takes photos as part of the process, at least in this case) and maps them to the collected points, giving us a true 3D representation of the space, which is the back of a theater by the way.
In the second I have included both the SLAM cloud and HDLS together, you can see where the SLAM backfilled around the objects and the cabinets, giving us data to model from, the extents of walls, heights and profiles of objects, etc.
The last is solely a SLAM cloud, I can tell what and where the objects are but the cloud is just a mass, no photos, and the details/density would not allow us to determine, let’s say, the cabinet handles. Do you need definition of handles? Or in most cases of documenting existing, do you just need a volumetrically correct model that looks good in elevation and model view. We’ve completed projects where the client wanted a 3D database of almost  everything because they were retrofitting an HVAC package into an existing and very significant building. They wanted to A) document everything properly and B) be able to fabricate any objects from the data in case they were damaged during construction. This was obviously a case where we needed to create high def scans through out the entire building, in classrooms though, which did not have significant architectural detail we used our own software, PKNail, to create the geometry. It’s the ability to blend technologies and capture methods that allows a service provider to best serve the client from a detail and budgetary point of view. After you’ve completed the data collection, made sure all the data is registered to each other, even from disparate sources, that is SLAM with HDLS, with Drone captured data, etc. you’ll need to choose whether you want Revit or Autocad…but that’s an entirely different conversation, however, more times than not when using these technologies together you are creating a robust 3D data base of the building that can be revisited for any questions or even new documentation needs.
everything because they were retrofitting an HVAC package into an existing and very significant building. They wanted to A) document everything properly and B) be able to fabricate any objects from the data in case they were damaged during construction. This was obviously a case where we needed to create high def scans through out the entire building, in classrooms though, which did not have significant architectural detail we used our own software, PKNail, to create the geometry. It’s the ability to blend technologies and capture methods that allows a service provider to best serve the client from a detail and budgetary point of view. After you’ve completed the data collection, made sure all the data is registered to each other, even from disparate sources, that is SLAM with HDLS, with Drone captured data, etc. you’ll need to choose whether you want Revit or Autocad…but that’s an entirely different conversation, however, more times than not when using these technologies together you are creating a robust 3D data base of the building that can be revisited for any questions or even new documentation needs.
| PRO | CON | |
| HDLS | Best granularity of data. Point density. | Almost all machines are stationary so every shot needs to be set up and registered. Although software advances have helped in automation. Slower process. |
| SLAM | Quick, captures data at walking speed. Decent density for details and over all dimensions. Covers areas well where it has range. | Limited range. Occasional hiccups and data “drift” |
| Drone | Quickest, Exterior Only, great for massing and building geometry. Capturing data in hard or inaccessible places. Captures hi-res images of project. | Limited data density to pick up details. Object distortion. |
Pros and Cons of SLAM / HDLS / Drone ©Pointknown 2018
Below are some screenshots that will put most to sleep but if you’ve read this far, maybe not. The large one is of combined clouds (HDLS / SLAM) of a building we completed in LA. They were registered together, and rendered in Revit / ReCap. The first of the smaller ones is a close up of a wall with just the SLAM cloud. The second is the HDLS data overlaid the wall in Revit, the last is just the data. You’ll notice how tight the HDLS Line, with very little spread between the data points. The SLAM data looks faded in comparison and the “spread” between the farthest data point and closest is about 1/2”, that is the HDLS line looks like it was drawing with a ball point pen, the SLAM with highlighter. Experience in  dealing with both sets, pro and cons can help
dealing with both sets, pro and cons can help .
. 

Pointknown Wraps Up 18 Tremont : Building Surveying & Documentation : Direct to Revit
Posted by Jim Foster in BIM, Built Environment, Revit on February 8, 2016
We recently delivered a Revit EB (Existing Building) Model of 18 Tremont a historic 12-story, 202,000-square-foot office with ground-floor retail in downtown Boston. The building was acquired by DLJ Real Estate Capital Partners in October 2015. Pointknown, with its partners, created an exterior HDLS (High Definition Laser Scan) and Revit model of the exterior as well as the elevator lobby, and stair cores. After creating the base Revit model the Pointknown Team utilized PKNail Pro , a point to point, direct to Revit, reality capture tool, to document interior wall partitioning, doors, and bathroom layouts. Using the combined technologies helped us tremendously in speed and accuracy, and the database functionality of Revit allowed us to assign spaces/offices and run space calculations a lot easier than polylining spaces. We were then able to produce formatted documents, floor plans, sections, space calculations for the owners / investors and delivered the model for the designers.
BIM As Built : Laser Technology
Posted by Jim Foster in As Builts, BIM, Laser Scanning, New Technologies, Revit on December 1, 2009
Speaking to a colleague from Europe who moved to the US because he stated, ‘there’s a survey shop on every corner in the UK, I could only find a handful here.’ However, that’s changing. HDLS, high definition laser scanning is starting to take off, especially now that the GSA issued the laser scanning awards. Additionally, more and more people are reworking existing assets / adaptive reuse projects so it is becoming more and more important to get the existing conditions data. While everyone is waiting for the day we can walk around with a magic wand and wave it around the room we have to build a bridge from here to there. HDLS in creating a 3D database is an excellent start. I believe HDLS, from firms like Leica and FARO, performs fantastically in certain circumstances such as inaccessible or difficult geometry, exposed MEP intensive projects, however, for typical conditions especially the interior of buildings it might be akin to using a sledgehammer rather when you need a fly swatter. We, PointKnown, have been developing a product that bolts on to Revit and takes laser range finder data and builds objects as you measure. This has been defined as PPLT (Point to Point Laser Technology). It allows a surveyor to move quickly and accurately from room to room or object to object. We do not intend for it to usurp laser scanning but rather augment current surveying teams, allowing them the most flexibility depending on the situation.
Most importantly is to define the deliverable and type of model needed for the project. This can start at the basic architectural model using generic library items to, well , anything goes but most of our clients want dimensionally correct space and then they apply the material and details they need as that is what they want to control.
We are now accepting people and firms into our public beta that starts January 2010, if you have any interest please feel free to contact us at info@pointknown.com and put beta in the subject line.